Since the early days of human civilization, gold has been valued as a precious metal. As early as 4000 BC, gold could be found in decorative objects and jewelry, and by around 1550 BC, it had become a medium of exchange to facilitate global trade.
Today, gold has a variety of applications, from electronics to being a protective hedge against inflation. To ensure it maintains a predictable and consistent value, however, gold must be refined. This article outlines the various refining processes to achieve the highest levels of purity.
Why Does Gold Need To Be Refined?
Many people are surprised to learn that when gold is mined from the ground, it contains several impurities. According to the World Gold Council, extracted gold ore is only about 60% to 90% pure. For savvy investors as well as various applications like medical devices and electronics, gold must be at purity levels of 99.99%, which is the threshold for 24-karat gold.
There are several reasons to refine gold, including:
- Removing natural impurities
- Combining to create gold alloys
- Meeting various end-use requirements
- Creating a suitable and consistent store of value
- Recycling for reuse
Depending on the refining process, different levels of purity can be achieved. For example, 22-karat gold is preferred for jewelry because it is more durable and easier to work with. 22-karat gold, however, has a purity level of approximately 91.7%.
How Gold Is Refined

Gold refining involves removing impurities from the metal, which is vital to achieving the desired level of purity for gold’s various applications.
Depending on the specific needs of the application for which the gold will be used, there are several refining options. Some are more exact than others, so different processes can be suitable when relatively higher levels of impurities are acceptable.
Fire Assay
Also referred to as cupellation, fire assay is not technically a refining process. Instead, as the term “assay” suggests, a fire assay is a more traditional method of measuring or testing the purity level of a gold or silver sample or scrap gold.
The sample is melted in a furnace, and the resulting molten ore is then poured into a cupel. A cupel is a small, porous cup that absorbs impurities, leaving behind a bead of gold that can be weighed. The weight of the bead is compared to the original weight of the sample to determine its purity.
Miller Process
Named for the process’s inventor, Francis Bowyer Miller, the Miller Process can refine gold up to a purity level of 99.5%. It’s ideal for applications that require refining large volumes of gold, and it involves several complex steps.
Like most gold refining processes, the Miller Process begins by melting the gold into doré bars. Doré bars are bricks of gold that are typically created at the site of a mine before being transported to a facility for further purification.
After melting the gold, the next step is to expose the doré bar to chlorine gas, which separates the gold from the impurities by binding them together. The remaining liquid condenses into a more pure form of gold that is suitable for bullion, some electrical components, and various other uses.
Aqua Regia

Aqua regia is a Latin term that means “royal water.” The aqua regia gold refining process involves the use of highly corrosive chemicals to allow gold ore to reach purity levels of 99.99%. The chemical combination includes the use of both nitric acid (HNO3) and hydrochloric acid (HCl) in a very specific ratio.
Due to the toxicity of the chemicals involved, there are several safety precautions that must be adhered to in order to successfully use this gold refining process. Due to the care involved, it is most suitable for laboratory applications to prepare samples.
Wohlwill Process
Like the aqua regia process, the Wohlwill process is known for creating the highest purity levels, reaching up to 99.999% pure gold.
The early stages of the Wohlwill process mirror the Miller process, beginning with melting gold bars. Then, an aqua regia solution is used to dissolve the bar. From there, an electrical process, referred to as electrolysis, occurs, which migrates impurities to an anode, leaving the purer form of gold in the cathode. The gold left in the cathode is further processed to reach even higher purity levels.
Though the Wohlwill process is not used as frequently as other gold refining techniques, it is often the preferred method when the highest levels of purity are required.
Carbon Absorption
Carbon absorption is a refining technique that’s not limited to precious metals. It is also used for purifying gases, liquids, and solutions.
The overall process involves absorbing carbon molecules onto an activated carbon surface, which is highly porous. Before this occurs, however, the gold is put in contact with a cyanide solution. The combined gold-cyanide solution bonds to the carbon and is transferred to a tank, where another solution strips the gold from the carbon.
When there are complex solutions, carbon absorption can be especially efficient. The process is also adaptable to a variety of refining environments.
Market and Industrial Applications of Refined Gold
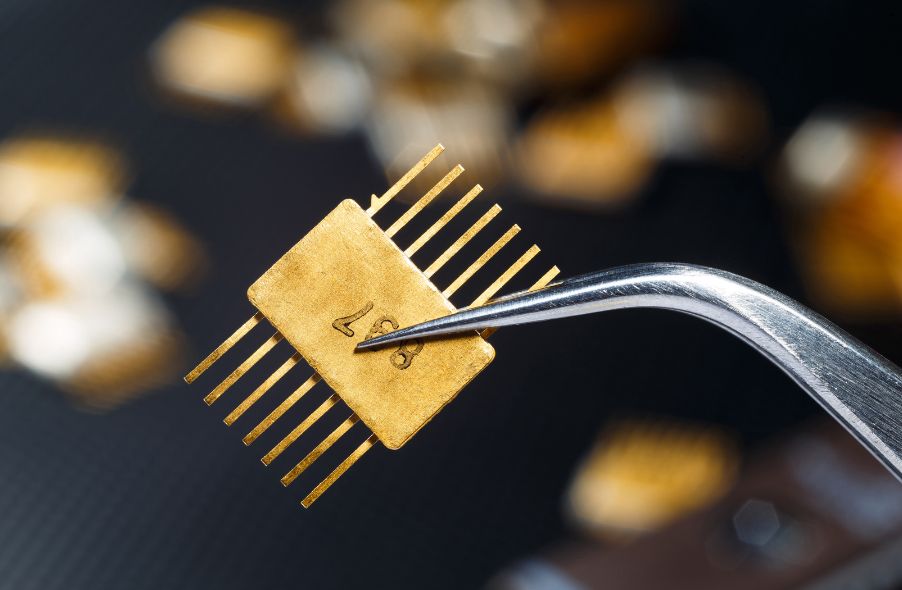
Aside from its monetary value, gold is a highly diverse metal that benefits a variety of applications, from adorning fingers and necklines to making up components in electrical devices like smartphones. It is adept at resisting the elements thanks to its inert properties.
In other words, gold resists rust, tarnish, and corrosion. It’s also highly malleable, even when not at its melting point. It is popular in electronic devices and medical equipment because it can conduct electricity, and its bioavailability makes it an ideal choice for human applications like dental implants, diagnostic tools, and more.
Jewelry Manufacturing
From a cultural standpoint, jewelry is one of the most popular and well-known uses for refined gold. The malleability, ductility, and inherent beauty of gold make it a preferred metal for crafting intricate and durable jewelry pieces.
Again, the biocompatibility of gold makes it ideal for bracelets, earrings, and other gold jewelry pieces that come in direct contact with the skin, making it less likely to cause allergies or skin reactions.
Electronics Industry
Gold is prevalent in the electronics industry, finding its way into the following types of devices:
- Computers
- Smartphones and tablets
- Circuit boards
- Telecommunications equipment
- Medical devices
- Aerospace electronics
- Audio equipment
- Automotive equipment
The list above is nowhere near exhaustive. The conductivity and corrosion resistance of the metal make it a logical choice for various electronic devices. Further, gold is common for thin film deposition applications, wiring, and plating.
Investment and Bullion
Gold is often purchased in bullion form as an investment vehicle and a hedge against inflation. It can also be useful in portfolio diversification, and even today, it can be used as a medium of exchange. Because it is recognized as a store of value on a global scale, gold gives people a true sense of security when looking for assets that have legitimate value.
Conclusion
Gold is an asset class that is well-suited to any investment portfolio. With an estimated 77% of the world’s gold already mined, this precious metal is expected to become even more scarce and valuable over time. To begin investing in gold or add more gold to your portfolio, contact Learn About Gold today.





